Analysis
Aluminum occurs in nutrient solutions primarily as the aluminum ion (Al³⁺) or as hydroxo complexes. Essential for some plants (e.g., peas, corn, sunflowers, and cereals). Can be toxic to some plants at concentrations above 10 ppm. Sometimes used to produce flower pigments (e.g., hydrangeas). Variable micronutrient.
There are different methods for determining aluminum:
- Complexometric titration with EDTA: Formation of a stable Al-EDTA complex.
- Spectrophotometry with eriochrome cyanine R: color development by complex formation.
- Atomic absorption spectroscopy (AAS): High-precision determination of aluminum.
Detailed titration of aluminum with EDTA
1. Principle of the method
Aluminum ions (Al³⁺) react with ethylenediaminetetraacetic acid (EDTA, C₁₀H₁₆N₂O₈) to form a stable chelate complex:
The endpoint of the titration is detected using the xylenol orange indicator. The color changes from yellow to red .
2. Chemicals
- 0.01 mol/L EDTA solution (C₁₀H₁₆N₂O₈)
- Buffer solution (pH 5, acetate buffer)
- Xylenol orange (indicator)
3. Experimental setup
Required equipment:
- Burette (25 mL, division 0.1 mL)
- Erlenmeyer flask (250 mL)
- Pipette (10 mL)
- Magnetic stirrer
4. Implementation
- Pour 10 mL of the nutrient solution into a 250 mL Erlenmeyer flask.
- Add 10 mL of acetate buffer solution (pH 5).
- Add 2-3 drops of xylenol orange indicator.
- Titrate with 0.01 mol/L EDTA until the color changes from yellow to red.
5. Calculation of the aluminum concentration
The concentration of Al³⁺ is calculated using the formula:
6. Example calculation:
- EDTA concentration: 0.01 mol/L
- Consumed volume: 7.8 mL (0.0078 L)
- Sample volume: 50 mL (0.050 L)
Conclusion
Complexometric titration with EDTA is a reliable method for the quantitative determination of aluminum in nutrient solutions.
Other names for xylenol orange:
-
- C31H32N2O13S
- C31H28N2Na4O13S (Tetranatriumsalz)
- 3,3-Bis(N,N-bis(carboxymethyl)aminomethyl)kresolsulfonphthalein
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

|
legend PA: Stands for "pro analysi" or "analytically pure" and means that the substance can be used for analytical procedures, as the content of foreign substances is specified. |
Chemicals and laboratory equipment required for nutrient analysis in hydroponic solutions
We recommend titration for home analysis. This is by far the most cost-effective method and, for occasional tests, probably the most sensible one in terms of cost and effort.
Here you will find a summary of the chemicals and laboratory equipment you will need to analyze the respective components in your nutrient solutions. The articles on the respective tests, with a detailed procedure for each "substance" and a sample calculation, can be found in the Analysis section .
Chemicals reference :
https://www.carlroth.com/de/de/massloesungen/massloesungen-utility-ready/c/web_folder_716991
https://www.laboratoriumdiscounter.nl/de/chemikalien/

Necessary material
Burette:
25ml in increments from 0.1ml to 0.05ml, available from Amazon to Aliexpress for prices ranging from approximately €25 to €660. The price difference also lies in the quality.
Magnetic stirrer + magnetic stir bar. No heating is necessary for our analyses :
Magnetic stirrers cost €30 to €300. You can also purchase them from lab supply retailers. These prices should be viewed with skepticism. They can exceed €1,000. Personally, I don't understand where the additional costs come from.
Erlenmeyer flask:
Approximately €5 to €50. The flask used for the analysis does not need to be heat-resistant (borosilicate glass/Pyrex), so you can also perform the titration in a drinking glass.
Necessary chemicals
Analysis of aluminum (Al)
Chemicals required:
- 0.01 mol/L EDTA solution (C₁₀H₁₆N₂O₈)
- Buffer solution (pH 5, acetate buffer)
- Xylenol orange (indicator)
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of arsenic (As)
Chemicals required:
- 0.01 mol/L iodine solution (I₂)
- 1 mol/L hydrochloric acid (HCl)
- 0.1 mol/L sodium thiosulfate solution (Na₂S₂O₃)
- Starch solution (indicator)
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of Lead (Pb)
Chemicals required:
- 0.01 mol/L EDTA solution (C₁₀H₁₆N₂O₈)
- Acetic acid/acetate buffer solution (pH 5-6)
- Xylenol orange (indicator)
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of boron (B)
Chemicals required:
- Sodium hydroxide (NaOH, 0.01 mol/L) – for the titration of boron.
- Mannitol – to form the boron-mannitol complex.
- Phenolphthalein – as an indicator for color detection.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of Calcium (Ca)
Chemicals required:
- EDTA (0.01 mol/L) – for titration of calcium.
- Eriochrome Black T – as an indicator for color recognition.
- Ammonia buffer solution (pH 10) – to stabilize the pH value.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of chlorine (Cl)
Chemicals required:
- Silver nitrate (AgNO₃, 0.01 mol/L) – for the precipitation of chloride as AgCl.
- Potassium chromate (K₂CrO₄) – as an indicator for Mohr titration.
- Nitric acid (HNO₃, 1 mol/L) – to control the pH value.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of iron (Fe)
Chemicals required:
- EDTA (0.01 mol/L) – for titration of iron.
- Xylenol orange – as an indicator for color recognition.
- Acetic acid/sodium acetate buffer solution (pH 5-6) – to control the pH value.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of Potassium (K)
Chemicals required:
- Sodium tetraphenylborate (Na[B(C₆H₅)₄]) – for the precipitation of potassium.
- Indicator (e.g. toluene extract) – for color detection.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of cobalt (Co)
Chemicals required:
- 0.01 mol/L EDTA solution (C₁₀H₁₆N₂O₈)
- Buffer solution (pH 10, NH₃/NH₄⁺ buffer)
- Eriochrome Black-T (indicator)
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of Copper (Cu)
Chemicals required:
- EDTA (0.01 mol/L) – for titration of copper.
- Indicator (e.g. Eriochrome Black T) – for color recognition.
- Ammonia buffer solution (pH 10) – to stabilize the pH value.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of lithium (Li)
Chemicals required:
- 0.01 mol/L ammonium tetraphenylborate solution (NH₄BPh₄)
- Ethanol-water mixture as solvent
- Phenolphthalein as a turbidity indicator
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of magnesium (Mg)
Chemicals required:
- EDTA (0.01 mol/L) – for titration of magnesium.
- Indicator (e.g. Eriochrome Black T) – for color recognition.
- Ammonia buffer solution (pH 10) – to stabilize the pH value.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of manganese (Mn)
Chemicals required:
- Potassium permanganate (KMnO₄, 0.01 mol/L) – for the titration of manganese.
- Sulfuric acid (H₂SO₄, 1 mol/L) – for dissolving manganese compounds.
- Indicator (e.g. Murexide) – for color detection.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of molybdenum (Mo)
Chemicals required:
- Iron(II) sulfate (FeSO₄, 0.01 mol/L) – for the titration of molybdenum.
- Sulfuric acid (H₂SO₄, 1 mol/L) – to control the pH value.
- Distilled water – for dilution.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of nickel (Ni)
Chemicals required:
- 0.01 mol/L EDTA solution (C₁₀H₁₆N₂O₈)
- Buffer solution (pH 9-10, NH₃/NH₄⁺ buffer)
- Murexide (indicator)
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of phosphorus (P)
Chemicals required:
- Lanthanum(III) chloride (LaCl₃, 0.01 mol/L) – for the titration of phosphate.
- Nitric acid (HNO₃, 1 mol/L) – to control the pH value.
- Sodium rhodizonate – as an indicator for color detection.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of sulfur (S)
Chemicals required:
- Barium sulfate solution (BaSO₄) – for precipitating the sulfur.
- Diluted HCl – for acid adjustment.
- Indicator (e.g. methyl orange) – for color detection during titrations.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of nitrogen (N)
Chemicals required:
- Formaldehyde (37% solution) – for complex formation with ammonium.
- HCl (0.01 mol/L) – for back titration.
- Indicator (e.g. Thoron) – for color detection of the endpoint.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of mercury (Hg)
Chemicals required:
- 0.01 mol/L dithizone solution (C₁₃H₁₂N₄S)
- Sulfuric acid (H₂SO₄, diluted)
- Chloroform (CHCl₃, for extraction)
- Buffer solution (pH 4-5)
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of silicon (Si)
Chemicals required:
- 0.01 mol/L sodium fluoride (NaF) solution
- 0.01 mol/L lanthanum (III) chloride (LaCl₃) solution
- Buffer solution (pH 3, acetic acid/sodium acetate buffer)
- Alizarin complexone (indicator)
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
Analysis of zinc (Zn)
Chemicals required:
- EDTA (0.01 mol/L) – for titration of zinc.
- Indicator (e.g. Eriochrome Black T) – for color recognition.
- Ammonia buffer solution (pH 10) – to stabilize the pH value.
Required laboratory equipment:
- burette
- Erlenmeyer flask
- pipette
- Magnetic stirrer
| All information is provided without guarantee. Please keep in mind that we may make typos! Quote:
Be careful when reading health books; the slightest typo can kill you. |
ID: 641
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

Arsenic (As) is a toxic metalloid that can be naturally occurring or enter drinking water through industrial processes. Long-term exposure to arsenic can lead to serious health problems such as skin lesions, cancer, and neurological disorders. First of all, only instrumental methods are suitable: HG-AAS, ICP-MS, etc.
Limit values for arsenic in drinking water
- Current limit in Germany (since 2013): 10 µg/L (0.01 mg/L) [Source]
- Planned limit value (from June 24, 2023): 4 µg/L (0.004 mg/L) [Source]
Qualitative detection reactions for arsenic
Various methods exist for the qualitative detection of arsenic in aqueous solutions. However, many of these traditional methods lack the sensitivity required to detect the low concentrations permitted in drinking water according to the above-mentioned limits.
1. Bettendorf test
Principle: Reduction of arsenic(III) ions by tin(II) chloride in hydrochloric acid solution, forming a brown precipitate of elemental arsenic.
Detection limit: The exact detection limit is not clearly documented, but is typically in the range of mg/L.
Assessment: Due to the relatively high detection limit, this method is not suitable for the detection of arsenic in drinking water below the legal limit values .
2. Gutzeit test
Principle: Formation of arsine (AsH₃) by reaction of arsenic with zinc and acid; AsH₃ reacts with silver nitrate paper to form a yellowish-brown stain.
Detection limit: This method is more sensitive than the Bettendorf assay, but may still have difficulty reliably detecting concentrations in the range of a few µg/L.
Assessment: Although more sensitive, this method is only partially suitable for the detection of arsenic in drinking water close to the current limit values .
More sensitive methods for trace analysis
Instrumental methods are used for the accurate determination of arsenic in drinking water:
- Atomic absorption spectroscopy with hydrogenating technique (HG-AAS): Very precise method for arsenic determination in trace levels.
- ICP-MS (Inductively Coupled Plasma Mass Spectrometry): Extremely sensitive, can detect arsenic in the ng/L range.
Conclusion
Most conventional qualitative detection methods, such as the Bettendorf or Gutzeit assay, are unsuitable for detecting arsenic in drinking water below legal limits due to their higher detection limits. Therefore, instrumental methods such as HG-AAS or ICP-MS are recommended for precise qualitative and quantitative determination of arsenic in drinking water.
ID: 678
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

Arsenic (As) is not found in any nutrient solutions. It occurs in the following forms: arsenite (As³⁺) and arsenate (As⁵⁺) . It is highly toxic.
The following methods are available for determination:
- Atomic absorption spectrometry (AAS) with hydride generator (HG-AAS): High sensitivity.
- Inductively coupled plasma mass spectrometry (ICP-MS): Very precise.
- Spectrophotometry with silver diethylthiocarbamate: color development by complex formation.
- Electrochemical methods (e.g. ASV): High sensitivity.
- Iodometric titration: Suitable for As³⁺.
Titration of arsenic with iodine solution (I₂)
1. Principle of the method
Arsenic(III) ions (As³⁺) are oxidized to arsenic(V) by iodine (I₂) in acidic solution:
The endpoint is detected using starch solution as an indicator ( blue → colorless ).
2. Chemicals
- 0.01 mol/L iodine solution (I₂)
- 1 mol/L hydrochloric acid (HCl)
- 0.1 mol/L sodium thiosulfate solution (Na₂S₂O₃)
- Starch solution (indicator)
3. Experimental setup
Required equipment:
- Burette (25 mL, division 0.1 mL)
- Erlenmeyer flask (250 mL)
- Magnetic stirrer
- Graduated pipettes (10 mL, 50 mL)
4. Implementation
- Add 10 mL of 1 mol/L HCl to 10 mL of nutrient solution.
- Carefully heat the solution to 40°C.
- Slowly add 0.01 mol/L iodine solution while stirring.
- After the yellow color disappears, add starch solution.
- Continue titrating until the blue color disappears.
5. Calculation of the arsenic concentration
The concentration of As³⁺ is calculated as follows
:
6. Example calculation
- Used iodine solution: 7.5 mL (0.0075 L)
- Concentration of iodine solution: 0.01 mol/L
- Sample volume: 50 mL (0.050 L)
Conclusion
Iodometric titration is a simple, cost-effective method for the quantitative determination of arsenic in nutrient solutions. Alternatively, AAS or ICP-MS offer greater accuracy.
ID: 666
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

The term borate species refers to the various chemical forms (species) in which boron can exist in a solution. The form depends strongly on the pH value .
Important borate species
1. Boric acid (H₃BO₃) – undissociated, neutral
- Predominant at pH < 7
- Acts as a weak Lewis acid
- Exists mainly as uncharged molecules
2. Tetrahydroxoborate ion ([B(OH)₄]⁻) – anionic
- Predominant at pH > 9
- Formed by the reaction of boric acid with hydroxide ions (OH⁻)
- Important for the titration of boron with NaOH
3. Polycondensed borates
- At higher concentrations and certain pH ranges, boron can form borate oligomers or polyborates
- An example is the tetraborate ion [B₄O₇]²⁻
4. Boron-mannitol complex
- By adding mannitol, boron forms a stable complex
- This complex acts like a strong acid
- Can be titrated by NaOH
Summary
| pH range | Predominant borate species |
|---|---|
| pH < 7 | Boric acid (H₃BO₃) |
| pH 7 – 9 | Equilibrium between H₃BO₃ and [B(OH)₄]⁻ |
| pH > 9 | Tetrahydroxoborate ion ([B(OH)₄]⁻) |
| With mannitol | Boron-mannitol complex (titratable with NaOH) |
ID: 638
Context:
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

Boron is present in nutrient solutions mainly as borate species (B(OH)₄⁻) .
There are various methods for determining boron:
- Spectrophotometry with azomethine-H: color development by complex formation.
- ICP-OES (Inductively Coupled Plasma with Optical Emission): High-precision determination.
- Manual titration with mannitol and NaOH: formation of a stable boron-mannitol complex.
Detailed titration of boron with mannitol and sodium hydroxide
1. Principle of the method
Boron forms a stable bormannitol complex with mannitol , which can be titrated as a strong acid:
The complex can then be titrated with sodium hydroxide (NaOH) .
2. Chemicals
- 0.01 mol/L sodium hydroxide solution (NaOH)
- Mannitol (C₆H₁₄O₆, as a reagent)
- Phenolphthalein (indicator)
3. Experimental setup
Required equipment:
- Burette (25 mL, division 0.1 mL)
- Erlenmeyer flask (250 mL)
- Pipette (10 mL)
- Magnetic stirrer
4. Implementation
- Pour 10 mL of the nutrient solution into a 250 mL Erlenmeyer flask.
- Add 5 g of mannitol and dissolve.
- Add 2-3 drops of phenolphthalein indicator.
- Titrate with 0.01 mol/L NaOH until the color changes from colorless to pink.
5. Calculation of the boron concentration
The concentration of B is calculated using the formula:
6. Example calculation:
- NaOH concentration: 0.01 mol/L
- Consumed volume: 6.3 mL (0.0063 L)
- Sample volume: 50 mL (0.050 L)
Conclusion
Titration with mannitol and NaOH is a simple procedure.
.
ID: 628
Kontext:
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

Cadmium (Cd) is a toxic heavy metal that can get into drinking water through industrial processes, agricultural activities or corrosion of pipes. It can cause kidney, liver and bone damage.
Limit values for cadmium in drinking water
- EU limit (according to Directive 98/83 / EC): 5 µg / L (0.005 mg / L) [Source]
- WHO guideline: 3 µg / L (0.003 mg / L) [Source]
- EPA (USA) limit: 5 µg / L (0.005 mg / L) [Source]
Qualitative detection reactions for cadmium
There are various methods for the qualitative detection of cadmium in aqueous solutions. However, it should be noted that many of these methods do not have the sensitivity required to detect the low concentrations that are permitted in drinking water according to the limit values mentioned above.
1. Detection with dithizone
Principle: With cadmium ions, dithizone forms a colored complex that shows an intense color.
Detection limit: The detection limit for cadmium with dithizone is approximately 3 µg / L (0.003 mg / L), which corresponds to the WHO guideline. [Source]
Rating: Suitable. Due to the sufficient sensitivity, the dithizone test can be used for the qualitative detection of cadmium in drinking water.
2. Detection with potassium iodide (KI test)
Principle: Cadmium ions react with potassium iodide to cadmium iodide under certain conditions.
Detection limit: The exact detection limit is not clearly documented, but is typically in the range of mg / L.
Rating: Due to the higher detection limit, this test for the detection of cadmium in drinking water is below the legal limit values not suitable.
3. Detection with sulfuric acid (H ₂ SO ₄ test)
Principle: Cadmium ions can react with sulfuric acid under certain conditions.
Detection limit: Similar to the AI test, the detection limit is in the range of mg / L.
Rating: Not sensitive enough for the detection of cadmium in drinking water below the legal limit values.
More sensitive methods for trace analysis
Instrumental methods are used for a more precise determination of cadmium in drinking water:
- Atomic absorption spectroscopy (AAS): Very precise method for traces of cadmium with a detection limit of up to 0.1 µg / L. (Source)
- ICP-MS (Inductively Coupled Plasma Mass Spectrometry): Extremely sensitive, cadmium can detect in the ng / L range.
- Anodic Stripping Voltammetrie (ASV): Electrochemical method for trace analysis.
Conclusion
The qualitative analysis of cadmium can be carried out with dithizone, since this test has sufficient sensitivity to detect the low concentrations that are permitted in drinking water according to the legal limit values. Other classic detection methods such as the potassium iodide or sulfuric acid test are not suitable for this purpose due to their higher detection limits. Instrumental methods such as AAS or ICP-MS are recommended for precise quantitative determination.
ID: 681
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

Cadmium (Cd) is primarily present as Cd²⁺ ions.
Alternative Methods for Cadmium Analysis
- Atomic Absorption Spectroscopy (AAS): High sensitivity and accuracy.
- Inductively Coupled Plasma Mass Spectrometry (ICP-MS): Very precise for trace analysis.
- Spectrophotometry with Dithizone: Color development through complex formation.
- Complexometric Titration with EDTA: Suitable for the quantitative determination of Cd²⁺.
Titration of Cadmium with EDTA
1. Principle of the Method
Cadmium ions (Cd²⁺) form a stable complex with Ethylenediaminetetraacetic Acid (EDTA, C₁₀H₁₆N₂O₈):
The endpoint is detected using Eriochrome Black T as an indicator (color change from wine red to blue).
2. Chemicals
- 0.01 mol/L EDTA solution (C₁₀H₁₆N₂O₈)
- Buffer solution (pH 10, NH₃/NH₄⁺ buffer)
- Eriochrome Black T (indicator)
3. Experimental Setup
Required Equipment:
- Burette (25 mL, 0.1 mL graduations)
- Erlenmeyer flask (250 mL)
- Pipette (10 mL)
- Magnetic stirrer
4. Procedure
- Add 10 mL of the nutrient solution to a 250-mL Erlenmeyer flask.
- Add 10 mL of buffer solution (pH 10).
- Add 2-3 drops of Eriochrome Black T indicator.
- Titrate with 0.01 mol/L EDTA until the color changes from wine red to blue.
5. Calculation of Cadmium Concentration
The Cd concentration is calculated using the formula:
6. Example Calculation
- EDTA concentration: 0.01 mol/L
- Volume used: 9.2 mL (0.0092 L)
- Sample volume: 50 mL (0.050 L)
Conclusion
The complexometric titration with EDTA is a precise method for the quantitative determination of cadmium in nutrient solutions. For more accurate trace analysis, AAS or ICP-MS is recommended.
ID: 669
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

Calcium occurs in nutrient solutions mostly as Ca²⁺ ion and can be determined using various methods:
- Complexometric titration with EDTA: Frequently used method.
- Atomic absorption spectroscopy (AAS): Very precise but expensive laboratory analysis.
- ICP-OES (Inductively Coupled Plasma with Optical Emission): High-precision method for multiple measurements.
Detailed titration of calcium with EDTA
1. Principle of the method
Calcium ions (Ca²⁺) react with ethylenediaminetetraacetic acid (EDTA, C₁₀H₁₆N₂O₈) to form a stable chelate complex:
The endpoint is detected using the Eriochrome Black T (indicator) , which changes from red to blue.
2. Chemicals
- 0.01 mol/L EDTA solution
- Eriochrome Black-T (indicator)
- Ammonia buffer solution (pH 10)
- Distilled water
3. Experimental setup
Required equipment:
- Burette (50 mL, division 0.1 mL)
- Erlenmeyer flask (250 mL)
- Pipette (10 mL)
- Magnetic stirrer
4. Implementation
- Pour 10 mL of the nutrient solution into a 250 mL Erlenmeyer flask.
- Add 5 mL of ammonia buffer solution (pH 10).
- Add 2-3 drops of Eriochrome Black-T as an indicator (color: red).
- Titrate with 0.01 mol/L EDTA solution until the color changes from red to blue .
5. Calculating the calcium concentration
The calcium concentration is calculated using the following formula:
6. Example calculation:
- EDTA concentration: 0.01 mol/L
- Consumed volume: 8.5 mL (0.0085 L)
- Sample volume: 50 mL (0.050 L)
Conclusion
Titration with EDTA is a reliable and cost-effective method for determining calcium in nutrient solutions.
ID: 627
Kontext:
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

Price list with sources for chemicals used for analysis. All chemicals used for nutrient analysis are listed here. Please note that prices may vary depending on the supplier, purity, and packaging size. The prices listed are guidelines and are subject to change daily. All prices exclude VAT. As of April 2025:
| chemical | concentration | Packaging size | Price | Source | link |
| EDTA solution (C₁₀H₁₆N₂O₈) | 0.01 mol/L | 1 L | 28.50 € | Carl Roth | link |
| Buffer solution (pH 5, acetate buffer) | - | - | - | - | - |
| Xylenol orange (indicator) | powder | 100 g | $48.25 | Fisher Scientific | link |
| Iodine solution (I₂) | 0.01 mol/L | 1 L | 174.25 € | Carl Roth | link |
| Hydrochloric acid (HCl) | 1 mol/L | 1 L | 24.20 € | Carl Roth | link |
| Sodium thiosulfate solution (Na₂S₂O₃) | 0.1 mol/L | 500 ml | JPY 1,500 (~$9.67) | AS ONE Corporation | link |
| Starch solution (indicator) | 1% in ethanol | 1 L | 52.90 € | Carl Roth | link |
| Sodium hydroxide (NaOH) | in cookies | 1 kg | 35.90 € | Carl Roth | link |
| L-Mannitol | 500 mg | - | 498.00 € | Carl Roth | link |
| Phenolphthalein (indicator) | 1% in ethanol | 220 ml | 26.90 € | Carl Roth | link |
| Eriochrome Black T (indicator) | - | 100 g | 64.90 € | Carl Roth | link |
| Ammonia buffer solution (pH 10) | 15% | 1 L | 20.50 € | Carl Roth | link |
| Silver nitrate (AgNO₃) | 0.01 mol/L | - | €78.70 | VWR International | link |
| Potassium chromate (K₂CrO₄) | ≥99%, pa, ACS | 100 g | 45.90 € | Carl Roth | link |
| Nitric acid (HNO₃) | 2 mol / 2 N | 500 ml | 35.90 € | Carl Roth | link |
| Sodium tetraphenylborate (Na[B(C₆H₅)₄]) | - | 10 g | $64.65 | Thermo Fisher Scientific | link |
| Ammonium tetraphenylborate solution (NH₄BPh₄) | - | - | - | - | - |
| Ethanol | ≥99.5%, Ph. Eur., pure | 2.5 L | 199,00 € | Carl Roth | link |
| Potassium permanganate (KMnO₄) | powder | 250g | 29.50 € | Carl Roth | link |
| Sulfuric acid (H₂SO₄) | 96% pure | 2.5 L | 50.50 € | Carl Roth | link |
| Murexide (indicator) | - | 50 g | 129.00 € | Carl Roth | link |
| Iron(II) sulfate (FeSO₄) | ≥99%, pa, ACS | 500g | 40.90 € | Carl Roth | link |
| Lanthanum(III) chloride (LaCl₃) | ≥99.9%, cryst. | 100 g | 79.90 € | Carl Roth | link |
| Sodium rhodizonate | 98.5%, per annum | 25 g | 139.00 € | Carl Roth | link |
| Barium sulfate solution (BaSO₄) | - | - | - | - | - |
| Formaldehyde (35% solution) | 35% | 5 L | 49.00 € | Fischar | link |
| Dithizone solution (C₁₃H₁₂N₄S) | - | 5 g | 42.90 € | Carl Roth | link |
| Chloroform (CHCl₃) |
99.8 atom%D, stabilized with Ag |
1 L | 77.90 € | Carl Roth | link |
| Sodium fluoride (NaF) solution | ≥99%, pa, ACS | 1 kg | 46.90 € | Carl Roth | link |
| Alizarin complexone (indicator) | - | 5 g | 9.90 € | S 3 Chemicals | link |
ID:
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

Chlorine is present in nutrient solutions mainly as chloride ion (Cl⁻) .
There are different methods for determining chlorine:
- Argentometric titration according to Mohr: precipitation titration with silver nitrate (AgNO₃).
- Ion chromatography: Very precise method for analyzing chloride.
- Potentiometric measurement with chloride-specific electrode: Direct determination via ion selectivity.
Detailed titration of chloride with silver nitrate (AgNO₃) – Mohr method
1. Principle of the method
Chloride ions (Cl⁻) react with silver nitrate (AgNO₃) to form silver chloride (AgCl), which precipitates as a white precipitate:
Potassium chromate (K₂CrO₄) serves as the indicator . After complete precipitation of AgCl, excess Ag⁺ is bound, forming red silver chromate (Ag₂CrO₄) , which indicates the endpoint.
2. Chemicals
- 0.01 mol/L silver nitrate (AgNO₃) solution
- Potassium chromate (K₂CrO₄) as an indicator
- Distilled water
- Nitric acid (HNO₃, if necessary for pH adjustment)
3. Experimental setup
Required equipment:
- Burette (25 mL, division 0.1 mL)
- Erlenmeyer flask (250 mL)
- Pipette (10 mL)
- Magnetic stirrer
4. Implementation
- Pour 10 mL of the nutrient solution into a 250 mL Erlenmeyer flask.
- Add 2-3 drops of potassium chromate indicator.
- Titrate with 0.01 mol/L AgNO₃ until the color changes from white (AgCl) to red (Ag₂CrO₄) .
5. Calculation of the chloride concentration
The concentration of Cl⁻ is calculated using the formula:
6. Example calculation:
- AgNO₃ concentration: 0.01 mol/L
- Consumed volume: 9.2 mL (0.0092 L)
- Sample volume: 50 mL (0.050 L)
Conclusion
The argentometric titration according to Mohr is a simple and precise method for the determination of chloride in nutrient solutions.
ID: 639
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

Cobalt occurs in nutrient solutions primarily as the cobalt(II) ion (Co²⁺) . Required by rhizobia, it is important for the nodulation of legumes. A non-essential micronutrient.
There are various methods for determining cobalt:
- Atomic absorption spectroscopy (AAS): High-precision determination of cobalt.
- Spectrophotometry with nitroso-R salt: formation of a colored cobalt complex.
- Complexometric titration with EDTA: Formation of a stable cobalt-EDTA complex.
Detailed titration of cobalt with EDTA
1. Principle of the method
Cobalt ions (Co²⁺) react with ethylenediaminetetraacetic acid (EDTA, C₁₀H₁₆N₂O₈) to form a stable complex:
The endpoint of the titration is detected using Eriochrome Black-T (ErioT) as an indicator. The color change occurs from **pink to blue**.
2. Chemicals
- 0.01 mol/L EDTA solution (C₁₀H₁₆N₂O₈)
- Buffer solution (pH 10, NH₃/NH₄⁺ buffer)
- Eriochrome Black-T (indicator)
3. Experimental setup
Required equipment:
- Burette (25 mL, division 0.1 mL)
- Erlenmeyer flask (250 mL)
- Pipette (10 mL)
- Magnetic stirrer
4. Implementation
- Pour 10 mL of the nutrient solution into a 250 mL Erlenmeyer flask.
- Add 10 mL of buffer solution (pH 10).
- Add 2-3 drops of Eriochrome Black-T indicator.
- Titrate with 0.01 mol/L EDTA until the color changes from pink to blue.
5. Calculation of the cobalt concentration
The concentration of Co is calculated using the formula:
6. Example calculation:
- EDTA concentration: 0.01 mol/L
- Consumed volume: 9.2 mL (0.0092 L)
- Sample volume: 50 mL (0.050 L)
Conclusion
Complexometric titration with EDTA is a precise method for the quantitative determination of cobalt in nutrient solutions.
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:

In environmental chemistry, chemical oxygen demand (COB) is a measure of the amount of oxygen that can be consumed by reactions in a measured solution. It is usually expressed as the mass of oxygen consumed relative to the volume of solution, which in SI units is milligrams per liter ( mg/L ). A COB test is a simple way to quantify the amount of organic matter in water. The most common use of COB is to quantify the amount of oxidizable pollutants in surface waters (e.g. lakes and rivers ) or wastewater. COB is useful in water quality because it provides a measure of the effect of a wastewater on the receiving body, similar to biochemical oxygen demand ( BOD ).
The basis of the COD test is that almost all organic compounds can be completely oxidized to carbon dioxide using a strong oxidizing agent under acidic conditions. The amount of oxygen required to oxidize an organic compound to carbon dioxide, ammonia and water is given by:
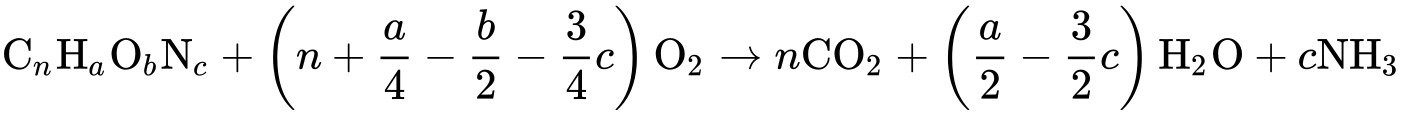
This expression does not take into account the oxygen demand due to nitrification, the oxidation of ammonia to nitrate:
![]()
Dichromate, the oxidizing agent used to determine COD, does not oxidize ammonia to nitrate. Therefore, nitrification is not included in the standard COD test.
The International Organization for Standardization describes a standard method for measuring chemical oxygen demand in ISO 6060 [1].
1) “General Chemistry Online: Glossary”: https://antoine.frostburg.edu/chem/senese/101/glossary/f.shtml
Source (own translation): https://en.wikipedia.org/wiki/Chemical_oxygen_demand
ID: 608
- Details
- Parent Category: Technology
- Category: Analysis
-
Also available:


