In environmental chemistry, chemical oxygen demand (COB) is a measure of the amount of oxygen that can be consumed by reactions in a measured solution. It is usually expressed as the mass of oxygen consumed relative to the volume of solution, which in SI units is milligrams per liter ( mg/L ). A COB test is a simple way to quantify the amount of organic matter in water. The most common use of COB is to quantify the amount of oxidizable pollutants in surface waters (e.g. lakes and rivers ) or wastewater. COB is useful in water quality because it provides a measure of the effect of a wastewater on the receiving body, similar to biochemical oxygen demand ( BOD ).
The basis of the COD test is that almost all organic compounds can be completely oxidized to carbon dioxide using a strong oxidizing agent under acidic conditions. The amount of oxygen required to oxidize an organic compound to carbon dioxide, ammonia and water is given by:
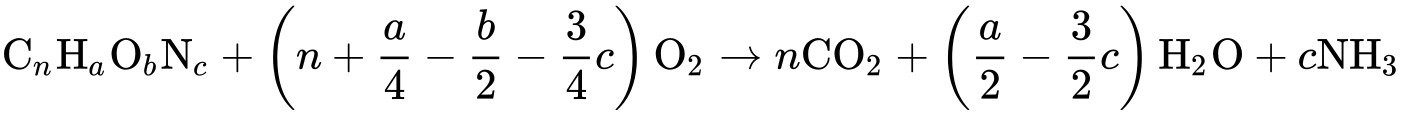
This expression does not take into account the oxygen demand due to nitrification, the oxidation of ammonia to nitrate:
![]()
Dichromate, the oxidizing agent used to determine COD, does not oxidize ammonia to nitrate. Therefore, nitrification is not included in the standard COD test.
The International Organization for Standardization describes a standard method for measuring chemical oxygen demand in ISO 6060 [1].
1) “General Chemistry Online: Glossary”: https://antoine.frostburg.edu/chem/senese/101/glossary/f.shtml
Source (own translation): https://en.wikipedia.org/wiki/Chemical_oxygen_demand
ID: 608



Add Comment