
experiments in his laboratory
Electric conductivity
Electrical conductivity , also known as conductivity or EC value (from English electrical conductivity ), is a physical quantity that indicates how strong the ability of a substance is to conduct electric current. This value, among many others, is used to control the fertilizer concentration in aquaponics and hydroponics.
Water / Nutrients – Conductivity EC
Water is an important building material for plant growth and provides the plant with moisture, necessary for metabolic processes. It is also a carrier of nutrients and contains dissolved oxygen. Important properties of water are hardness, salt content, pH and alkalinity. The proportion of dissolved minerals is checked by measuring the electrical conductivity (EC - electrical conductivity), given in µS/cm, sometimes also in mS/cm (1000 µS/cm = 1 mS/cm).
The right nutrient selection and the right amount are important. In order to avoid under- or over-fertilization, the nutrient content is checked by measuring the electrical conductivity (EC). The higher the salt content, the higher the conductivity. The following definition is “arbitrary” but widely used.
Soft water – approx. 0 – 140 µS/cm
Hard water - > 840 µS/cm
As we can see, water already contains a certain amount of dissolved nutrients , depending on its hardness . The missing nutrients are added via hydroponic fertilizer . Fewer nutrients are needed at the beginning of growth and in the final stages.
A conductivity between approx. 1000 – 2000 µS/cm covers pretty much all needs. As an average guideline value, we consider 1500 µS/cm to be a working value (experience values). But it is always important to observe the plants.
Manufacturers of hydroponic fertilizers provide information on dosage and conductivity values, depending on the growth stage.
The higher the temperature, the lower the oxygen content in the nutrient solution:
| Temperature (°C) | Dissolved oxygen in water (mg/l) |
| 10 | 11.30 |
| 15 | 10.00 |
| 20 | 9.00 |
| 25 | 8.30 |
| 30 | 7.60 |
| 35 | 7:00 |
| 40 | 6.40 |
| 45 | 6:00 am |
pH value hydroponics - approx. pH 6.2
The acidity (pH value) of the water influences the availability of nutrients for the plants. A wide range of nutrients can best be absorbed by the roots in a pH range of 5.5 - 6.5 , regardless of the cultivation method.
The pH should be measured and adjusted to create favorable growth conditions. Since the plants do not like a pH change that is too rapid, the pH value adjustment should be done gradually.
The following rounded minimums and maximums from the 4 nutrient formulas are good guidelines for your own hydroponic nutrient solution:
| element | mg/l = ppm |
| Nitrogen (N) | 170 – 235 |
| Phosphorus (P) | 30 – 60 |
| Potassium (K) | 150 – 300 |
| Calcium (Ca) | 160 – 185 |
| Magnesium (Mg) | 35 – 50 |
| Sulfur (S) | 50-335 |
| Iron (Fe) | 2.5 – 12 |
| Manganese (Mn) | 0.5 – 2.0 |
| Copper (Cu) | 0.02 – 0.1 |
| Zinc (Zn) | 0.05 – 0.1 |
| Molybdenum (Mo) | 0.01 – 0.2 |
| boron (B) | 0.3 – 0.5 |
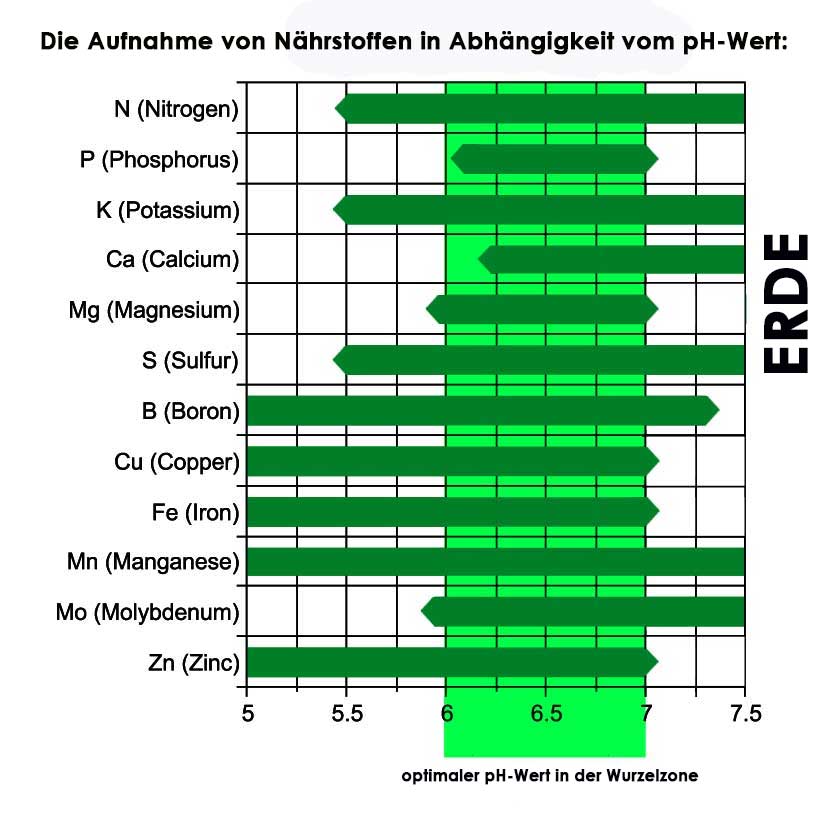
When growing hydroponically, it is advisable to allow the pH to fluctuate slightly within 6-7 pH. As you can see in the figure, some nutrients can only be absorbed at the lower or upper range of the optimal range.
 |
 |
Herbs for growing in hydroponics
Herbs thrive in hydroponics, grow very well and you can grow many herbs in a small space. If, as with classic cultivation in soil, you take into account the requirements for sun, partial shade or shade and keep an eye on the water-nutrient mixture, you can look forward to a rich harvest.
Regular pruning also promotes plant growth. The list shows herbs that are well suited for hydroponic cultivation, but does not claim to be complete.
- valerian
- basil
- Savory
- Borage
- Watercress
- Calendula
- dill
- Echinacea
- Angelica
- tarragon
- fennel
- Goldenseal
- chamomile
- Catnip
- chervil
- coriander
- cumin
- lavender
- Lovage
- dandelion
- marjoram
- Mint, all varieties
- Feverfew
- oregano
- Parsley
- Pimpinelle
- peppermint
- Rue / rocket
- rosemary
- sage
- chives
- Cut celery
- Stevia
- thyme
- Thai basil
- Wormwood
- Hyssop (verbena)
- Lemon basil
- Lemongrass
- Lemon balm
Vegetables for growing in hydroponics
Actually, you can grow almost all plants hydroponically, except root vegetables. Fast-growing varieties such as pak choi, Asian lettuce or chard are interesting because they can be harvested frequently. But many other types of vegetables also deliver high yields quickly and taste very good at the same time. The list shows examples of which vegetables can be cultivated hydroponically.
- eggplant
- Asian salad
- cauliflower
- Beans
- Broccoli
- chili
- endive salad
- Peas
- Strawberries
- Green mustard
- Kale
- Cucumbers
- Kohlrabi
- herb
- pumpkin
- Leek
- Chard
- Melons
- Mizuna - Japanese salad
- okra
- Pak choi
- paprika
- Brussels sprouts
- Red mustard
pH value and EC value for crops
| plant | pH | E.C |
|---|---|---|
| Broad bean | 6.0-6.5 | 1.8-2.2 |
| pineapple | 5.5-6.0 | 2.0-2.4 |
| artichoke | 6.5-7.5 | 0.8-1.8 |
| aubergine | 5.5-6.5 | 2.5-3.5 |
| banana | 5.5-6.5 | 1.8-2.2 |
| basil | 5.5 - 6.5 | 1.0-2.0 |
| Blueberry | 4.0-5.0 | 1.8-2.0 |
| Blueberry/blueberry | 4.0-5.0 | 1.8-2.0 |
| cauliflower | 6.0-7.0 | 0.5-2.0 |
| Beans | 6.0-6.5 | 1.8-2.5 |
| broccoli | 6.0-6.5 | 2.8-3.5 |
| chicory | 5.5-6.0 | 2.0-2.4 |
| chili | 5.8-6.3 | 1.8-2.8 |
| pea | 6.0-7.0 | 0.8-1.8 |
| strawberry | 5.5-6.5 | 1.8-2.2 |
| fennel | 6.4-6.8 | 1.0-1.4 |
| Cucumber | 5.8-6.0 | 1.7-2.5 |
| Ginger | 5.8-6.0 | 2.0-2.5 |
| Carrots | 6.3 | 1.6-2.0 |
| Potato | 5.0-6.0 | 2.0-2.5 |
| Garlic | 6.0 | 1.4-1.8 |
| Cabbage | 6.5-7.0 | 2.5-3.0 |
| cress | 6.0-6.5 | 1.2-2.4 |
| pumpkin | 5.5-7.5 | 1.8-2.4 |
| Leek | 6.5-7.0 | 1.4-1.8 |
| lavender | 6.4-6.8 | 1.0-1.4 |
| marjoram | 6.0 | 1.6-2.0 |
| Chard | 6.0-7.0 | 1.8-2.3 |
| melon | 5.5-6.0 | 2.0-2.5 |
| mint | 5.5-6.0 | 2.0-2.4 |
| Pak choi | 7.0 | 1.5-2.0 |
| paprika | 6.0-6.5 | 1.8-2.8 |
| Pepperoni | 6.0 | 1.4-1.8 |
| Pepperoni | 6.0-6.5 | 1.8-2.8 |
| Parsley | 5.5-6.0 | 0.8-1.8 |
| radish | 6.0-7.0 | 1.6-2.2 |
| rhubarb | 5.0-6.0 | 1.6-2.0 |
| Brussels sprouts | 6.5-7.5 | 2.5-3.0 |
| rosemary | 5.5-6.0 | 1.0-1.8 |
| Beetroot | 6.0-6.5 | 0.8-5.0 |
| Red currant | 6.0 | 1.4-1.8 |
| arugula | 6.0-7.5 | 0.8-1.2 |
| salad | 5.5-6-5 | 0.8-1.5 |
| sage | 5.5-6.5 | 1.0-1.6 |
| chives | 6.0 - 6.5 | 1.8-2.4 |
| Blackcurrant | 6.0 | 1.4-1.8 |
| celery | 6.5 | 1.8-2.4 |
| Spanish pepper | 6.0-6.5 | 1.8-2.2 |
| asparagus | 6.0-6.8 | 1.4-1.8 |
| spinach | 6.0-7.0 | 1.8-2.3 |
| Turnip | 6.0-6.5 | 1.8-2.4 |
| Sweet Granadilla | 6.5 | 1.6-2.4 |
| thyme | 5.5-7.0 | 0.8-1.6 |
| tomatoes | 5.5-6.5 | 1.5-2.5 |
| Watermelon | 5.8 | 1.5-2.4 |
| Lemon balm | 5.5-6.5 | 1.0-1.6 |
| zucchini | 6.0 | 1.8-2.4 |
| Onions | 6.0-6.7 | 1.2-1.8 |
pH values and EC values for ornamental plants
| Plant | PH value | EC value |
|---|---|---|
| Benjamini (Ficus) | 5.5-6.0 | 1.6-2.4 |
| Flower tube | 6.5-7.0 | 2.5-3.0 |
| Chrysanthemums | 6.0-6.2 | 1.8-2.5 |
| Dahlias | 6.0-7.0 | 1.5-2.0 |
| Dieffenbachia | 5.0 | 1.8-2.0 |
| Dragon trees | 5.0-6.0 | 1.8-2.4 |
| Ferns | 6.0 | 1.6-2.0 |
| window leaves | 5.0-6.0 | 1.8-2.4 |
| Busy Lizzie | 6.4-6.8 | 1.0-1.4 |
| Freesias | 6.5 | 1.0-2.0 |
| Plant | PH value | EC value |
|---|---|---|
| Common chicory | 5.5-6.0 | 2.0-2.4 |
| Gerberas | 5.0-6.5 | 2.0-2.5 |
| Gladioli | 5.5-6.5 | 2.0-2.4 |
| Hibiscus | 6.0-7.0 | 1.2-1.5 |
| Boat orchids | 5.5 | 0.6-1.0 |
| Caladiums | 6.0-7.5 | 1.6-2.0 |
| Clove | 6.0 | 2.0-3.5 |
| Roses | 5.5-6.0 | 1.5-2.5 |
| Violet | 6.9-7.0 | 1.2-1.5 |
EC values for hemp plants


Composition of a hydroponic nutrient solution (standard nutrient solution)
In hydroponic science, extensive research has been and is being carried out to find the best nutrient solution. Four standard nutrient formulas from Hoagland & Arnon (1938), Hewitt (1966), Cooper (1979) and Steiner (1984) are particularly well known. These are general standard nutrient solutions.
Here you will find a short introduction to how you can create nutrient solutions yourself.
The following rounded minimums and maximums from the 4 nutrient formulas are good guidelines for your own hydroponic nutrient solution:
| Element | mg/l = ppm |
| Nitrogen (N) | 170 – 235 |
| Phosphorus (P) | 30 – 60 |
| Potassium (K) | 150 – 300 |
| Calcium (Ca) | 160 – 185 |
| Magnesium (Mg) | 35 – 50 |
| Sulfur (S) | 50-335 |
| Iron (Fe) | 2.5 – 12 |
| Manganese (Mn) | 0.5 – 2.0 |
| Copper (Cu) | 0.02 – 0.1 |
| Zinc (Zn) | 0.05 – 0.1 |
| Molybdenum (Mo) | 0.01 – 0.2 |
| Boron (B) | 0.3 – 0.5 |
pH of the nutrient solution and nutrient availability
In order for your plant to grow and thrive in hydroponics, your nutrient solution must have a certain pH value. If the pH value is too high or too low, important nutrients are not available to the plant.
In most cases, the ideal pH value of the nutrient solution is between 5.5 – 6.5 . Most nutrients are available in this area. If you want to perfect yield and growth, you should find out about specific pH values for plants in hydroponics. Here is a diagram about the pH value and the availability of nutrients:

Graphic: Pennsylvania State University
Context:
ID: 162











