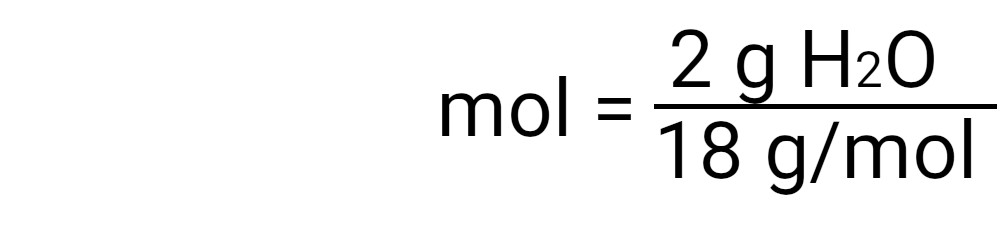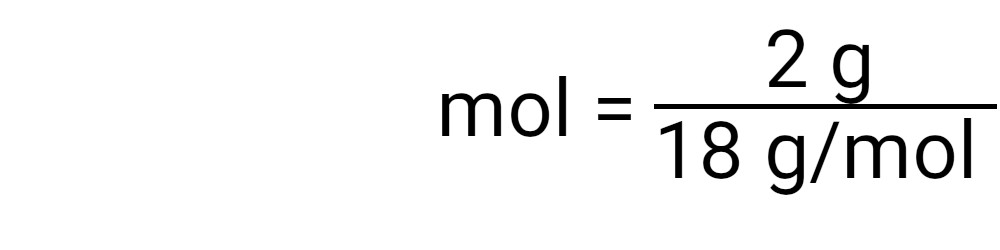|
Here we explain how grams are converted to moles. The conversion from moles to grams can be found here. This area of chemistry is called stoichiometry . You will need a periodic table and a calculator. First, identifying the elements that make up the compound.
Then determine the number of atoms each element contributes to the compound. Example: H 2 O has two hydrogen and one oxygen atoms. If an index follows a bracket in a compound, each element in the bracket is multiplied by the index. For example, (NH 4 ) 2 S consists of two nitrogen atoms, one hydrogen atom and one sulfur atom. Record the atomic weight of each element. A periodic table is the easiest way to determine the atomic weight of an element. Once you've found the element on a periodic table, the atomic weight is usually listed below the element symbol. For example, the atomic weight of oxygen is 15.99. Calculate Molecular Mass: The molecular mass of a substance is calculated by multiplying the number of atoms of each element by its respective atomic weight. To convert grams to moles, you need to know the molecular mass of the compound. Multiply the number of atoms of each element by its atomic weight. Example:
First step Second step Result
(NH 4 ) 2 S has the molecular mass of (2 x 14.01) + (8 x 1.01) + (1 x 32.07) = 68.17 g/mol. Molecular mass was previously also referred to as molecular weight. The number of moles in a compound can be calculated by dividing the number of grams of the compound by the molecular mass of the compound.
Once you have set up the formula, you can insert the calculations into the appropriate place in the formula. An easy way to check that everything is in the right place is to look at the units. You should be able to reduce all units so that only moles remain. Divide the number of grams by the molecular mass. The result is the number of moles in your element or compound. |
Context:
ID: 545