First we look at the nutrient solutions, some of which have been around for over a hundred years. This shows us in which concentrations the measurement must take place.
This serves as an initial orientation as to what nutrients or elements must be contained in a solution. A further step is to closely observe plant growth in order to be able to identify deficits as such.
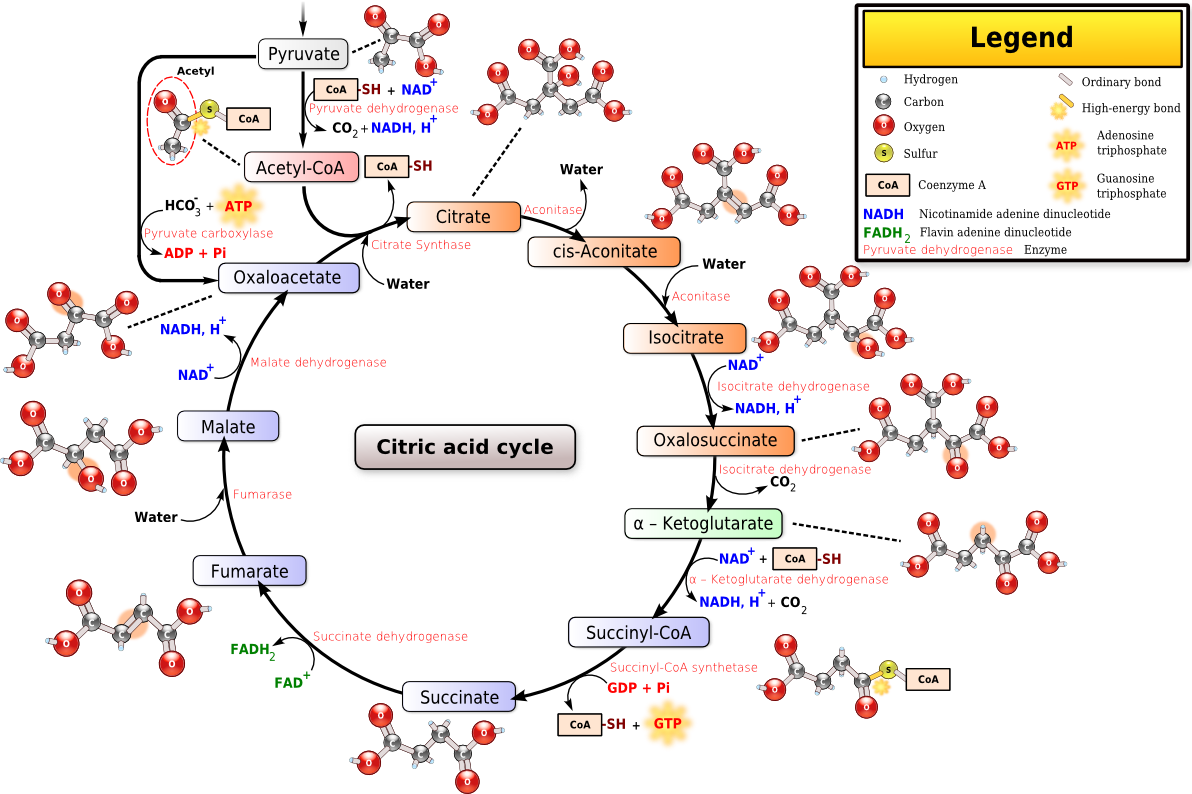
The next step is to get an idea of which elements, and therefore which compounds, are in the end product. Unfortunately, such an analysis (the plant is put into a blender and additional chemicals are added depending on the compounds we are looking for) has the disadvantage that it doesn't really reveal everything that interests us. This is because the chemical compounds can rarely be found in the plant in the form in which they were originally added. This is where biology comes into play. The only example that we would like to mention here is the citric acid cycle, which we do not want to withhold from you. It illustrates the complexity of metabolism.
Nutrition of hydroponic plants
Compounds and trace elements / orders of magnitude in nutrient solutions |
||
|
K |
Potassium |
0.5 - 10 mmol/L |
|
Approx |
Calcium |
0.2 - 5 mmol/L |
|
S |
Sulfur |
0.2 - 5 mmol/L |
|
P |
Phosphorus |
0.1 - 2 mmol/L |
|
Mg |
Magnesium |
0.1 - 2 mmol/L |
|
Fe |
Iron |
2 - 50 µmol/L |
|
Cu |
Copper |
0.5 - 10 µmol/L |
|
Zn |
Zinc |
0.1 - 10 µmol/L |
|
Mn |
Manganese |
0 - 10 µmol/L |
|
B |
Boron |
0 - 0.01 ppm |
|
Mo |
Molybdenum |
0 - 100 ppm |
|
NO2 |
Nitrite |
0 – 100 mg/L |
|
NO3 |
Nitrate |
0 – 100 mg/L |
|
NH4 |
ammonia |
0.1 - 8 mg/L |
|
KNO3 |
Potassium nitrate |
0 - 10 mmol/L |
|
Ca(NO3)2 |
Calcium nitrate |
0 - 10 mmol/L |
|
NH4H2PO4 |
Ammonium dihydrogen phosphate |
0 - 10 mmol/L |
|
(NH4)2HPO4 |
Diammonium hydrogen phosphate |
0 - 10 mmol/L |
|
MgSO4 |
Magnesium sulfate |
0 - 10 mmol/L |
|
Fe-EDTA |
Ethylenediaminetetraacetic acid |
0 – 0.1 mmol/L |
|
H3BO3 |
Boric acid |
0 – 0.01 mmol/L |
|
KCl |
Potassium chloride |
0 – 0.01 mmol/L |
|
MnSO4 |
Manganese (II) sulfate |
0 – 0.001 mmol/L |
|
ZnSO4 |
Zinc sulfate |
0 – 0.001 mmol/L |
|
FeSO4 |
Iron(II) sulfate |
0 – 0.0001 mmol/L |
|
CuSO4 |
Copper sulfate |
0 - 0.0002 mmol/L |
|
MoO3 |
Molybdenum oxide |
0 – 0.0002 mmol/L |
Here are some recipes for nutrient solutions...
1.00 g Ca(NO 3 ) 2 calcium nitrate
0.25 g MgSO 4 * 7 H 2 O magnesium sulfate
0.25 g KH 2 PO 4 potassium dihydrogen phosphate
0.25 g KNO 3 potassium nitrate
traces of FeSO 4 * 7 H2O iron(II) sulfate
1.5 millimol KH 2 PO 4
2.0 mM KNO 3
1.0 mM CaCl 2
1.0 mM MgSO 4
18 μM Fe-Na-EDTA
8.1 μM H 3 BO 3
1.5 μM MnCl2 _
1 mM KNO 3
1 mM Ca(NO 3 ) 2
1 mM NH 4 H 2 PO 4
1 mM (NH 4 ) 2 HPO 4
1 mM MgSO 4
0.02 mM Fe-EDTA
0.025 mM H 3 BO 3
0.05 mM KCl
0.002 mM MnSO 4
Trace elements:
0.002 mM ZnSO 4
0.0005 mM CuSO 4
0.0005 mM MoO 3
55 mg Al 2 (SO 4 ) 2
28 mg KJ 28 mg
KBr
55 mg TiO 2
28 mg SnCl 2 · 2 H 2 O
28 mg LiCl
389 mg MnCl 2 · 4 H 2 O
614 mg B(OH ) 3
55 mg ZnSO 4
55 mg CuSO 4 · 5 H 2 O
59 mg NiSO 4 · 7 H 2 O
55 mg Co(NO 3 ) 2 · 6 H 2 O
Context: